

The Aufbau Principle, also called the building-up principle, states that electron's occupy orbitals in order of increasing energy. The word 'Aufbau' is German for 'building up'. Notice that each subshell can only contain the max amount of electrons as indicated in the table above. If you count up all of these electrons, you will see that it adds up to 53 electrons. Its complete electron configuration is 1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 5. Using our example, iodine, again, we see on the periodic table that its atomic number is 53 (meaning it contains 53 electrons in its neutral state). Total number of possible electrons in each orbitalħ (f z 3, f xz 2, f xyz, f x(x 2-3y 2 ), f yz 2, f z(x 2-y 2), f y(3x 2-y 2) The following table shows the possible number of electrons that can occupy each orbital in a given subshell. Before continuing, it's important to understand that each orbital can be occupied by two electrons of opposite spin (which will be further discussed later). There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund's Rule and the Pauli-Exclusion Principle. Although the Schrödinger equation for many-electron atoms is extremely difficult to solve mathematically, we can still describe their electronic structures via electron configurations. Hence, the previously described postulate breaks down in that the energy of the electron is now determined by both the principal quantum number, n, and the orbital angular momentum quantum number, l. When dealing with multi-electron systems, we must consider the electron-electron interactions. This postulate, however, holds true only for Bohr's hydrogen atom or other hydrogen-like atoms. Therefore, the 3s orbital ( l=0) has the same energy as the 3p ( l=1) and 3d ( l=2) orbitals, regardless of a difference in l values. Out of these four quantum numbers, however, Bohr postulated that only the principal quantum number, n, determines the energy of the electron. No two paired electrons can have the same spin value. In general, an electron with a m s=+1/2 is called an alpha electron, and one with a m s=-1/2 is called a beta electron. Due to the spinning of the electron, it generates a magnetic field. The value of 1/2 is the spin quantum number, s, which describes the electron's spin.
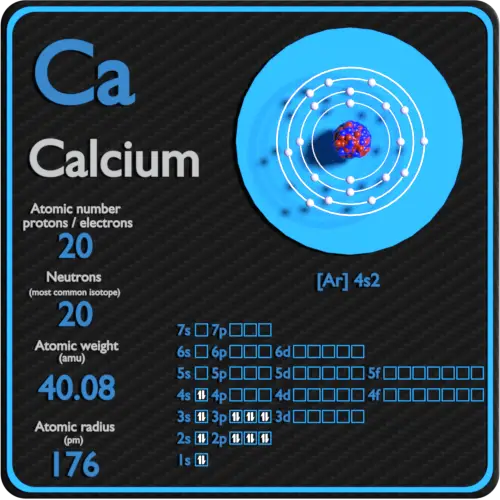
The spin magnetic quantum number can only have a value of either +1/2 or -1/2. These numbers can be thought of as an electron's "address" in the atom. Together, these four quantum numbers can be used to describe the location of an electron in Bohr's hydrogen atom. There is a fourth quantum number, called the spin magnetic quantum number (m s), which is not obtained from solving the Schrödinger equation. By solving the Schrödinger equation for the hydrogen atom, we obtain three quantum numbers, namely the principal quantum number (n), the orbital angular momentum quantum number ( l), and the magnetic quantum number (m l). The wavefunction is the solution to the Schrödinger equation. When assigning electrons to orbitals, we must follow a set of three rules: the Aufbau Principle, the Pauli-Exclusion Principle, and Hund's Rule. Hence, many of the rules that we use to describe the electron's address in the hydrogen atom can also be used in systems involving multiple electrons. In doing so, we obtain three quantum numbers (n, l,m l), which are the same as the ones obtained from solving the Schrödinger's equation for Bohr's hydrogen atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. The electron configuration is the standard notation used to describe the electronic structure of an atom.


 0 kommentar(er)
0 kommentar(er)
